Sister chromatids are equally distributed into two daughter cells during mitosis to ensure faithful transmission of genetic materials. Defects in segregation of sister chromatids lead to aneuploid or polyploid cells, which are intermediates in the progression of many human solid tumors and hallmarks of reproductive diseases and aging. Although various mechanisms to prevent mitotic errors have been reported, more regulatory pathways remain to be characterized.
A collaborative study led by Dr. ZANG Jianye, Dr. FU Chuanhai and Dr. YAO Xuebiao of the University of Science and Technology of China has revealed the molecular mechanism underlying the CENP-C-dependent kinetochore recruitment of Mis12 complex, which is negatively regulated by Aurora B-dependent phosphorylation of CENP-C and involved in spindle assembly checkpoint/error correction pathways. Their work establishes a previously uncharacterized regulatory mechanism involved in CENP-C–Mis12-facilitated kinetochore attachment error correction to ensure accurate chromosome segregation during mitosis. This work was published in Proc. Natl. Acad. Sci. U. S. A..
Faithful chromosome segregation during mitosis requires the accurate interaction of kinetochore with microtubule in a bi-polar manner. Spindle assembly checkpoint/error correction pathways monitor the connection between kinetochore and microtubule to ensure proper kinetochore-microtubule attachment. Previous work showed that Aurora B kinase is one of the essential components of these pathways, which phosphorylates the outer kinetochore proteins to promote the establishment of appropriate kinetochore-microtubule attachments. However, none of the inner kinetochore proteins has been shown as the substrate of Aurora B and involved in spindle assembly checkpoint/error correction pathways.
Researchers determined the crystal structure of Mis12-Nnf1 complex from the fission yeast Schizosaccharomyces pombe. Amino acid residues 26–50 of Cnp3 (the CENP-C homolog of S. pombe) was mapped as the minimal region responsible for direct binding of Cnp3 to Mis12-Nnf1 complex. Furthermore, Ark1 (the Aurora B homolog of S. pombe) phosphorylates Thr28 of Cnp3 to disrupt the interaction between Cnp3 and Mis12 complex. Phosphorylation of CENP-C by Aurora B kinase destabilizes kinetochore–microtubule attachment at the interface between the inner and outer kinetochore, facilitating kinetochore-microtubule attachment error correction.
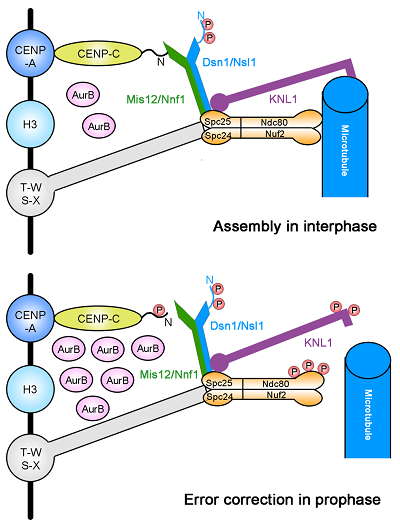
Image povided by Author
The study was supported by the Ministry of Science and Technology, the Chinese Academy of Sciences, and the National Science Foundation of China.
(School of Life Science and Hefei National Laboratory for Physical Sciences at Microscale)