Recently, researchers from University of Science and Technology (USTC) of Chinese Academy of Sciences (CAS) reported a universal strategy to fabricate single-atom catalysts (SACs). They synthesized more than thirty different SACs with 3d to 5d metal centers on several substrates via electrochemical deposition. The detailed research was published on Nature Communicationson entitled Electrochemical deposition as a universal route for fabricating single-atom catalysts on March 5th.
For the last decade, SACs have received wide attentions in catalyzing water splitting, oxygen reduction, CO2 hydrogenation, methane conversion, etc.due to their maximized atom utilizations and unique electronic structures. Despite that various strategies have been developed to fabricate SACs, these strategies generally have special requirements on the anchored metal or the supports. It remains a challenge to develop a universal approach that is applicable to a wide range of metals and supports for the fabrication of SACs.
The researchers conducted the electrochemical deposition of SACs in a standard three-electrode system. By tuning the potential range on the working electrode, two different Ir single atoms anchored on Co(OH)2 nanosheets (Ir1/Co(OH)2) were obtained from both cathodic and anodic electrodeposition. XAFS results revealed that these two Ir1/Co(OH)2 showed different valence states and coordination environments, which should be ascribed to different depositing species and the redox process on the electrode. Furthermore, they investigated the effects of the concentration of metal precursors, the number of scanning cycles, and the scanning rate on the formation of SACs during both cathodic and anodic electrodeposition. The results indicated that controlling the mass loading of metal species below a certain level is crucial for synthesizing SACs (Fig. 1). The upper limit of mass loading for SACs corresponds to the level of minimum supersaturation on the support, which is similar to the molecular mechanism of nucleation in the solution-phase synthesis. In the following, the researchers successfully deposited 4d and 5d metals on Co(OH)2 nanosheets, 3d metals on nitrogen-doped carbon, and Ir single atoms on different substratesto test the generality of this method. The single dispersion of metal species was validated by structural characterizations. In the meanwhile, the same type of SACs from cathodic and anodic electrodeposition also showed different electronic structures, holding potentials in application for different catalytic reactions.
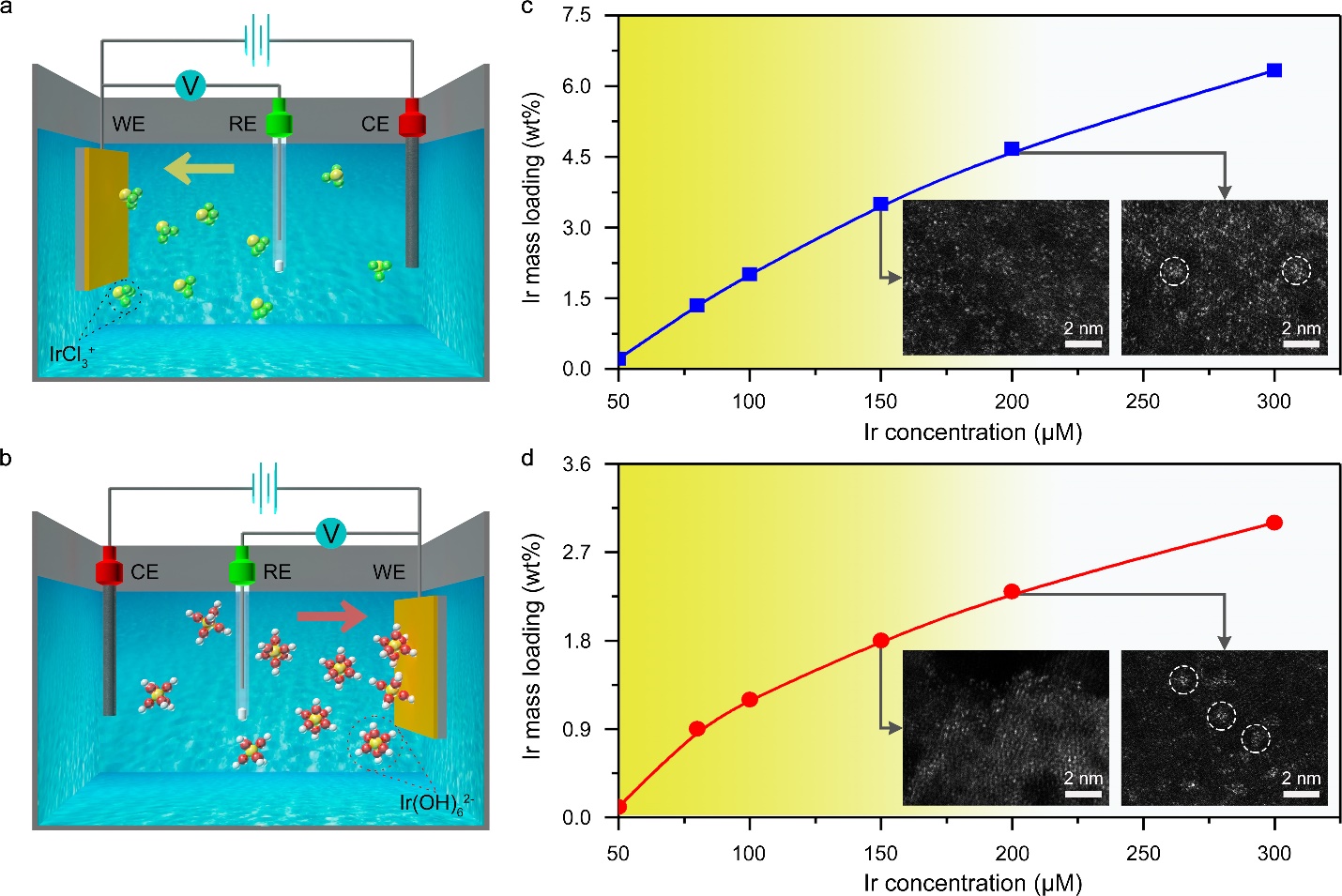
Electrochemical deposition mechanism. a, b, Schematic of cathodic (a) and anodic (b) deposition of Ir species. The yellow, green, red, and white spheres represent Ir, Cl, O, and H atoms, respectively. c, d, Ir mass loadings as a function of Ir concentration in the 1 M KOH electrolyte for cathodic (c) and anodic (d) deposition.
The as-obtained SACs were applied to catalyze water splitting. Cathodically deposited Ir single atoms on Co0.8Fe0.2Se2 nanosheets exhibited a current density of 10 mA cm-2 with only an overpotential of 8 mV for hydrogen evolution reaction, while anodically deposited Ir atoms also showed excellent performance for oxygen evolution reaction. Further, researchers assembled cathodically and anodically deposited Ir single atom into a two-electrode cell for overall water splitting. In order to increase the catalysts loading for better performance, the single atoms were grown on Ni foam. The electrochemical measurements suggested that only a record-low potential of 1.39 V was needed for a current density of 10 mA cm-2.
The generality of this method provides not only an easy access to a wide range of SACs, but also new pathways into indepth understanding of catalytic mechanisms.
PaperLink: https://www.nature.com/articles/s41467-020-14917-6.pdf
(Edited by LU Hongyu, USTC News Center)