The see-saw dilemma between the activity and selectivity of a catalyst in certain reactions has been bothering scientists for decades, since it’s hard to find a solution to boost the reaction rate while maintaining a high selectivity.
Recently, Prof. LU Junling and Prof. LI Weixue from the University of Science and Technology of China (USTC) focused on the selective hydrogenation reaction of halonitrobenzenes (HNBs), and jointly solved this prominent issue by designing and synthesizing a core–shell bimetallic Au@Pt/SiO2 catalyst with a monolayer platinum shell (Au@1ML-Pt). This work was published in Nature Catalysis.
Metal nanoparticle (NP) catalysts are widely used in various catalytic reactions, while the size of the particles greatly affects their performance, specifically their activity and selectivity. The tight coupling between the geometric and electronic effects related to the particle size tends to restrict the optimization of the catalyst’s performance, and, in many catalytic reactions, results in a see-saw relationship between activity and selectivity. Therefore, it remains a challenge to design and synthesize a catalyst that achieves high chemoselectivity and high activity simultaneously.
In this work, researchers first found that Pt/SiO2-catalyzed hydrogenation of HNBs into haloanilines (HANs) showed a see-saw relationship in its activity and selectivity. Then they pointed out that the lattice-stretch and the shift of the 5d-band center of Pt, caused by placing a monolayer of Pt(111) on the surface of Au(111), was capable of raising the catalyst’s activity and sustaining a high selectivity. It gave a feasible idea on how to break through the see-saw relationship. Based on the theoretical predictions, they precisely fabricated the Au@Pt/SiO2 catalyst with Au@1ML-Pt, using the unique technique of atomic layer deposition (ALD). Further characterizations were made and the results showed that the geometric and electronic structure of Pt was well-modulated in the bimetallic catalyst.
The Au@1ML-Pt catalyst also behaved exceedingly well in catalyzing the hydrogenation of HNBs. It showed an over 99% selectivity and a relatively high activity, resulting in a far better yield of HANs in the product than Au-Pt alloy or monometallic Pt catalyst. Therefore, the Au@1ML-Pt catalyst successfully achieved high activity and high selectivity at the same time, thus breaking through the see-saw dilemma.
The model of a core–shell bimetallic catalyst with a monolayer metallic shell holds unique characteristics in its geometric and electronic structures. And it will provide a promising strategy in designing high-activity, high-selectivity metallic catalysts in the future.
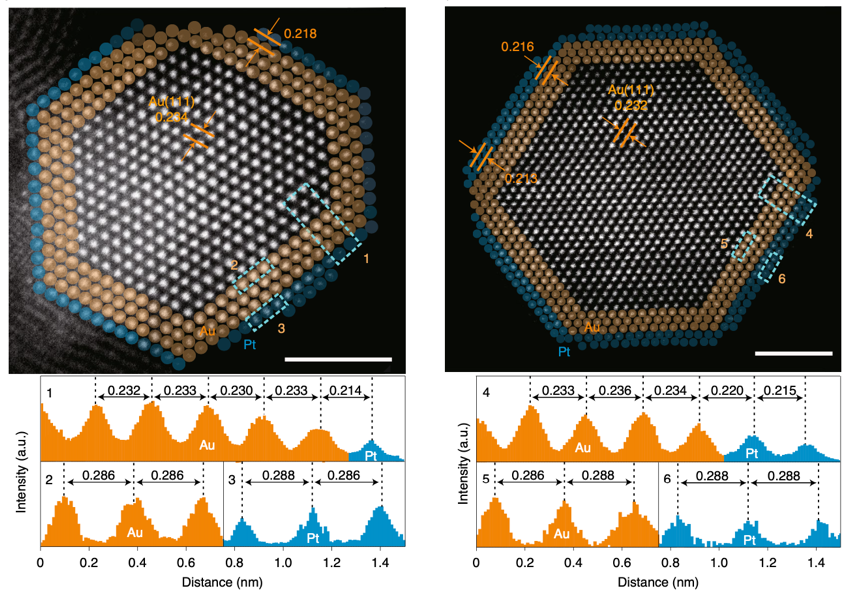
Representative atomic-resolution HAADF-STEM image of Au@1ML-Pt. (Image by GUAN Qiaoqiao et al.)
(Written by MENG Junyang, edited by WANG Zhaokun, USTC News center)