Hydrogenation of CO2 into fuels and useful chemicals serves as an important process which helps to alleviate the dearth of fossil fuels. As diversified reaction paths co-exist over practical catalysts towards CO2 hydrogenation, it is highly desiderated to precisely control the reaction path for developing efficient catalysts.
The research group of Prof. ZENG Jie from the University of Science and Technology of China, cooperated with Prof. SI Rui from Shanghai Synchrotron Radiation Facility, has made a great breakthrough in the hydrogenation of CO2 into methanol via single-atom catalysis.
The researchers reported that the ensemble of Pt single atoms coordinated with oxygen atoms in MIL-101 (Pt1@MIL) induces distinct reaction path to improve selective hydrogenation of CO2 into methanol. Pt1@MIL achieves the turnover frequencty number of 117 h-1 in DMF under 32 bar at 150 oC, which is 5.6 times that of Ptn@MIL. Moreover, the selectivity for methanol is 90.3% over Pt1@MIL, much higher than that (13.3%) over Ptn@MIL and that (76.2%) over Cu/ZnO/Al2O3 commercial catalyst.
The researchers found that every Pt single atom and its coordinated O atoms composed an active center in Pt1@MIL. The cooperativity between Pt single atoms and their coordinated O atoms in Pt1@MIL enabled the dissociation of H2 to form O-H groups. The hydroxy H atoms added into CO2 to produce HCOO* as the intermediates. The unique reaction path over Pt1@MIL not only lowered the activation energy for the enhanced catalytic activity, but also contributed to the high selectivity for methanol.
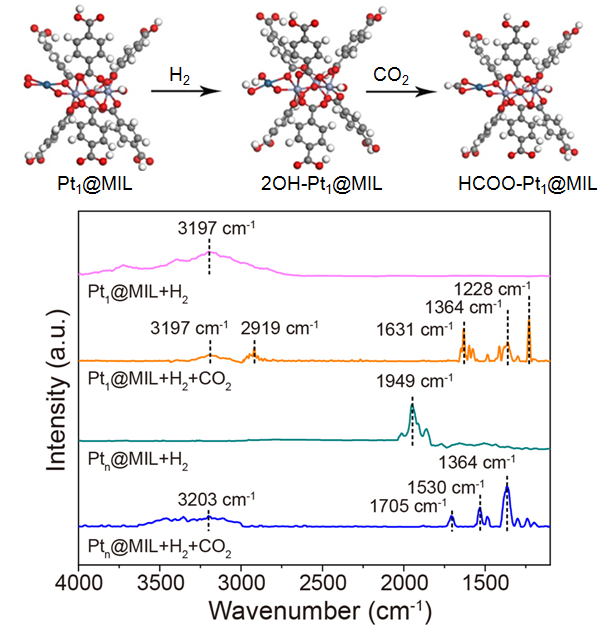
Figure. Reaction path of CO2 hydrogenation over Pt1@MIL. (Provided by professor ZENG’s team)
This work offers a powerful means to improve the catalytic performance for CO2 hydrogenation, and extends our understanding of single-atom catalysis. “Upgrading the catalysts from nanocrystals to single atoms not only enhances the atomic utilization efficiency, but also alters the catalytic mechanisms such as the adsorption of reactants or intermediates on catalysts and the reaction path.” said Ph.D. Chen.
Nevertheless, the development of CO2 hydrogenation is still on the way. “Despite of the deepened mechanistic understandings and breakthroughs in catalytic performance, challenges still remain in the field of CO2 hydrogenation. These challenges include searching the alternative source of hydrogen, exploring other routes, and so on.” said Prof. Zeng.
https://www.nature.com/articles/s41467-019-09918-z#auth-1
(Edited by FAN, School of Chemical Sciences)