Hydrogen energy has been intensely investigated as an ideal alternative to the conservative fossil fuels. Because it has high gravimetric energy density but no emission and it is abundant on earth. Mass hydrogen production could conveniently be realized by alkaline water electrolysis. Unfortunately, water splitting is not favored kinetically and hence requires highly active electrocatalysts to be expedited. Currently, the scalable hydrogen production is still difficult to be commercialized via electrocatalytic water splitting, limited by the lack of active and cheap hydrogen evolution electrocatalysts in basic media.
Prof. CHEN Qianwang's group has made progress in electrochemical hydrogen evolution electrocatalysts in basic media. By using Ru-doped Prussian blue analogues nanoparticles as precursors, researchers adopted a one-step thermal decomposition strategy for the fabrication of high-efficient and stable electrocatalysts composed of ruthenium and cobalt bimetallic nanoalloy encapsulated in nitrogen-doped graphene layers.
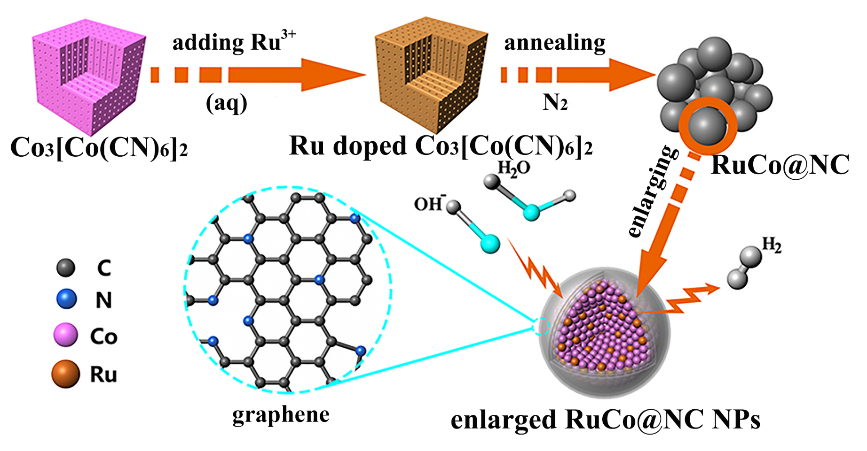
Figure. Schematic illustration of the synthetic route and model of the RuCo nanoalloys encapsulated in nitrogen-doped graphene layers (Image by CHEN Qianwang's group)
The catalysts display remarkable performance with low overpotentials of only 28 mV and 218 mV at 10 mA cm-2 and 100 mA cm-2, respectively, and excellent stability of 10 000 cycles. Ruthenium is the cheapest Platinum group metal and its amount in the catalyst is only 3.58 wt.%, showing the catalyst high activity at a very competitive price. Density functional theory calculations reveal that the introduction of ruthenium atoms into Cobalt core can improve the efficiency of electron transfer from alloy core to graphene shell, beneficial for enhancing carbon-hydrogen bond, thereby lowering △GH* of hydrogen evolution reaction.
The work provides a new way for the development of high-performance HER electrocatalysts in alkaline media while reducing the cost of noble metal electrocatalysts.
This work was recently published in Nature Communications entitled as “Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media” on April 25th.Graduate students Su Jiawei and Yang Yang contributed equally to this work. This work was supported by National Natural Science Foundation, CAS/ SAFEA International Partnership Program, and Fundamental Research Funds for the Central Universities.
The link of the paper: https://www.nature.com/articles/ncomms14969
(Hefei National Laboratory for Physical Sciences at the Microscale, School of Chemistry and Materials Science)
Contact
CHEN Qianwang
cqw@ustc.edu.cn
http://fnl.ustc.edu.cn/