A kid like his parents in looks is the result of inheriting faithful and reliable genetic information, DNA, from his parents which requires precise coordination and regulation of chromosome replication. This process requires the assembly of a replication complex, a primosome, at the origin. Without precise assembly, we may get some disease.
Prof.
TENG Maikun and Dr. LI Xu from USTC together with their team illustrated crystal structure of DnaT
84-153-dT
10 single stranded DNA (ssDNA) complex and revealed a novel single stranded DNA binding mode in the assembly process of replication. The research provides a deep insight into the mechanism of primosome assembly and stalled replication fork restart entitled “
Crystal structure of DnaT84–153-dT10 ssDNA complex reveals a novel single-stranded DNA binding mode” on July 22’s
Nucleic Acids Research.
The replication process is of vital significance to reliable transmission of genetic material. The primosome, required for assembly of a replication complex at the origin, consists of helicase, primase and several auxiliary proteins that are responsible for creating RNA primers on ssDNA during DNA replication. Accurate assembly is necessary for both normal chromosomal replication and the stalled replication fork restart.
Escherichia coli has two types of primosomes responsible for normal chromosomal replication and the stalled replication fork restart respectively. For the primosome, PriA-PriB-ssDNA-DnaT, it mainly starts the process of repairing damaged DNA with stalled replication fork. Researchers from USTC identified found that DnaT can bind to phiX 174 ssDNA to form nucleoprotein filaments for the first time with binding assays and negative-staining electron microscopy results, indicating that DnaT might function as a scaffold protein during the PriA-dependent primosome assembly. Combined with a series of biochemical analysis, they proposed how DnaT bind to ssDNA and the function of DnaT during the primosome assembly and stalled replication fork restart. It helps to explain the interactions between the three-helix bundle and ssDNA.
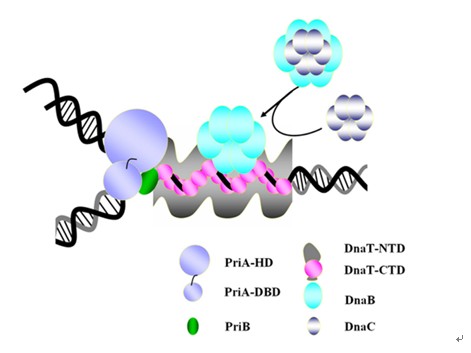
Fig: Model for DnaT facilitating PriA-dependent primosome assembly.
(JI Jiaojiao, USTC News Center)