If you exhibit symptoms of nausea, vomiting, diarrhea, loss of appetite, abdominal cramps and mild fever, don’t let down your guard. All the signs indicate that you may get infected with Staphylococcus aureus (S. aureus, also called golden staph).
For the past 20 years, S. aureus has long been recognized as one of the most important bacteria that cause community-associated and hospital-acquired infections in humans. It is the leading cause of skin and soft tissue infections such as abscesses (boils), furuncles, and cellulitis.
For the moment, effective measures have taken to preventstaph infections based on the studies of the spread of golden staph and the mechanism of how golden staph is capable of producing wound infections. Most infections caused by golden staph are treatable with antibiotics. However, there is a strong possibility that a few bacteria will survive a course of antibiotics. The antibiotic-resistant golden staph bacteria that remain then flourish, since they no longer have to compete for resources with the rest of the colony.
Antibiotic resistance is a serious public health problem and it’s urgent to develop new approaches to bring staphylococcal infections under control. YANG Yihu and JIANG Yongliang, from the lab of Prof. ZHOU Congzhao and Prof. CHEN Yuxing of University of Science and Technology of China, illustrated how Staphylococcus aureus adhere to host cells and provided us a way out of the mess.
To cause infection, a bacteria needs to first gain access to the host. This is preceded by attaching to the host cells or tissues. S. aureus has numerous surface proteins that promote attachment to host proteins. The research work depicted a surface-exposed serine-rich repeat glycoprotein (SRRP), which is involved in the pathogenesis of infective endocarditis via its ligand-binding region (BR) adhering to human platelets, and illustrated how SraP interacts with its host receptor(s). They have discovered a specific binding of SraP to N-acetylneuraminic acid (Neu5Ac) through structural and functional analyses of the BR domain. Further mutagenesis analysis showed that SraP binding to Neu5Ac and the trisaccharide promotes S. aureus adhesion to and invasion into host epithelial cells.
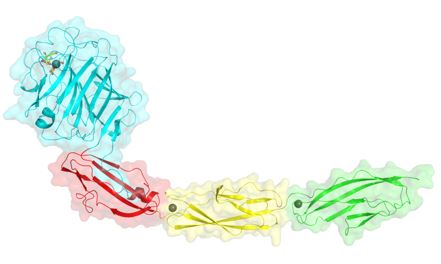
Figure. The intermodule twist along the axis of SraPBR.
The research findings lead scientists to find a new way to fight the infections and give us a deeper insight into developing Staphylococcus aureus vaccine or antibiotics. The research paper entitled “Structural Insights into SraP-Mediated Staphylococcus aureus Adhesion to Host Cells” was published on line as the Featured Research on PLOS Pathogens on June 5, 2014.
(JI Jiaojiao, USTC News Center)