Dr. YE Shuji group of Prof. LUO Yi Research Team from Hefei National Laboratory for Physical Sciences at the Microscale (HFNL) and Department of Chemical Physics at University of Science and Technology of China (USTC) made a new progress on the characterization of interfacial protein secondary structures using nonlinear vibrational spectroscopy recently. Dr. Ye’s group demonstrated for the first time that sum frequency generation vibrational spectroscopy(SFG-VS) can unambiguously differentiate the interfacial protein secondary structures by combining surface-sensitive amide I and amide III spectral signals. This combination offers a powerful tool to directly distinguish random-coil (disordered) and α-helical structures in proteins. This progress has been published in J. Am. Chem. Soc. 2014, 136(4), 1206-1209 titled as “Accurate Determination of Interfacial Protein Secondary Structure by Combining Interfacial-Sensitive Amide I and Amide III Spectral Signals”. It is another important progress of Dr.Ye’s group since they observed a model ion channel gating action in model cell Membranes in real time in situ using nonlinear SFG-VS(refer to: J. Am. Chem. Soc. 2012, 134, 6237−6243, Times cited: 23).
Structure determination holds the key for understanding and controlling the functionality of biological systems. With very limited tools at hand, there is an ever-growing demand for new and better experimental methods to accurately determine the structures, particularly the interfacial structures, of the proteins. The lack of surface-sensitive and label-free techniques has made it virtually impossible to explicitly distinguish the interfacial protein secondary structures. Consequently, many scientific problems that are associated with the identification of protein structures at the interface remain elusive. Precise molecular details of the interfacial protein structures are not only of scientific interest but also essential for the development of novel biomaterials and for finding effective treatments for “protein deposition diseases” such as Alzheimer’s, Parkinson’s, and prion diseases. These diseases are known to originate from the conformational transition of proteins at the interface. Prior to this work, the amide I vibration is the only one that has been used for secondary-structure analysis in previous SFG-VS studies. In this study, Dr.Ye’s group carefully examined the relevance of surface-sensitive SFG-VS for the determination of interfacial protein secondary structures. An experimental protocol that combines amide I and amide III spectral signals is successfully developed to accurately differentiate random-coil and α-helical structures at the interface, resolving an important, long-standing problem in interfacial protein science.
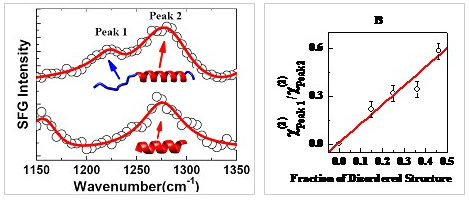
Figure 1、SFG spectra of interfacial protein in the amide III region and a linear correlation between the peak amplitude ratio and the content ratio of the disordered structure is clearly observed.
In addition, in terms of the symmetry constraints and chiral theory of SFG-VS, Dr. Ye’ group have successfully exploited SFG-VS to track the organization and transport of cholesterol in membrane by combining achiral-sensitive ssp (ppp) and chiral-sensitive psp polarization measurements. It is found that 6-KC molecules are aligned at outer leaflet of the DMPC lipid bilayer with a tilt angle of about 10°. 6-KC organizes itself by forming a-bstructure at low 6-KC concentration and most likely b-b structure at high 6-KC concentration. Among all proposed models, the results favor the so-called umbrella model with formation of 6-KC cluster. Moreover, they have found that the long anticipated flip-flop motion of 6-KC in membrane takes time to occur, at least much longer than previously thought. All these interesting findings indicate that it is critical to explore in situ, real-time, and label-free methodologies to obtain a precise molecular description of cholesterol’s behavior in membrane. This study represents the first application of SFG to reveal the cholesterol-lipid interaction mechanism at the molecular level and was published in J. Phys. Chem. Lett. 2014, 5, 419–424.
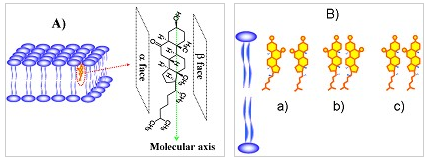
Figure 2、A) Molecular structure and the defined molecular axis of 6-KC; B) Three possible forms of side-by-side organizations of 6-KC.
The proceeding works were supported by the Ministry of Science and Technology of China, and the National Natural Science Foundation of China.
(Hefei National Laboratory for Physical Sciences at the Microscale)