Tocilizumab is an effective method for treating severe COVID-19 patients. Prof. XU Xiaoling, a respiratory and critical medical expert from the First Affiliated Hospital of USTC, and Prof. WEI Haiming from the Department of Life and Medical Sciences found that Tocilizumab can improve the clinical symptoms of COVID-19 patients. The research results were published on the Proceedings of the National Academy of Sciences (PNAS) on April 29 under the title of Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Prof. XU Xiaoling and Prof. HAN Mingfeng from Fuyang Second People's Hospital are the first authors of this paper, and Prof. WEI Haiming and Prof. XU Xiaoling are the corresponding authors.
In the research, 21 patients who were the first to use Topiramab in Fuyang Second People's Hospital and the First Affiliated Hospital of USTC were observed. The patients ranged in age from 25 to 88, including 17 severe cases and 4 critical cases. Their first symptom was fever and the average temperature was 38.8 ± 0.6 ℃. There was a 1-week history of routine treatment before the use of Topiramab, but the symptoms did not improve. After 5.6 days on average, all the patients had different degrees of deterioration with sustained fever and hypoxemia. CT of lung showed the progress of lesions. Among them, 2 cases were intubated and 1 case was non-invasive. On the first day after the treatment with Topiramab, the body temperature of all patients returned to normal, then remained stable, and the clinical symptoms were relieved significantly in the following days. The respiratory function of most patients was improved to some extent, and the chest tightness was relieved. 75% of the patients decreased the oxygen flow rate within 5 days, and the oxygen saturation of the peripheral blood remained stable. Two patients were evacuated from the ventilator within 5 days after receiving Topiramab. Most of the inflammatory indexes such as the number of peripheral blood lymphocytes and C-reactive protein recovered within 5 days. All patients have been discharged from the hospital, and the average hospitalization time after using Tuozhumab is 15.1 days. There were no adverse drug reactions and secondary pulmonary infection during the treatment.
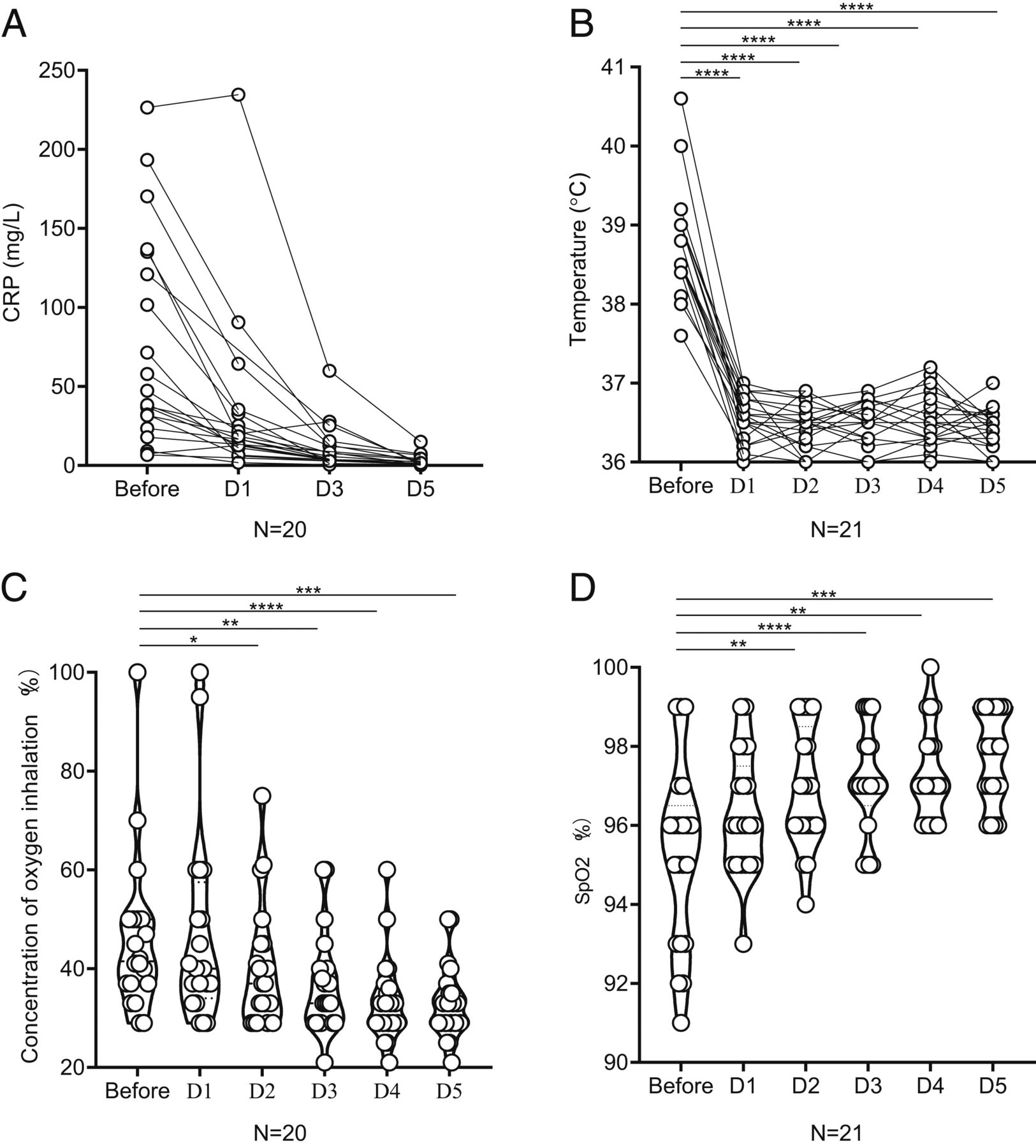
The research retrospectively analyzed the effect of Topiramab in the treatment of severe COVID-19 patients, and observed whether IL-6 played a key role in the pathogenesis and the therapeutic effect of Topiramab on IL-6, which provided a new effective treatment of severe COVID-19 patients.
PNAS magazine published relevant news reports on the research results.
Paper Link: https://www.pnas.org/content/early/2020/04/27/2005615117
(Written by YANG Xinqi, edited by LU Hongyu, USTC News Center. Materials Provided by the First Affiliated Hospital of USTC and the Department of Life and Medical Sciences)