With a high energy density, Li-O2 batteries have become a state-of-the-art battery technology. Inside the Li-O2 battery, the generation and disintegration of the discharged product solid lithium peroxide (Li2O2) have a significant effect on the battery’s performance. Previous researches shed little light on Li2O2 ‘s form and distribution inside, leaving questions regarding the trend and contributing factor of internal Li2O2 ‘s change in form and size unanswered.
Recently, a team led by Prof. TAN Peng from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences designed a carbon-coated anodic aluminum oxide (C-AAO) air electrode with a highly-ordered, array-like structure. The team gained new insights into the sudden death and reaction routes of Li-O2 batteries. The work was published in Nano Letters.
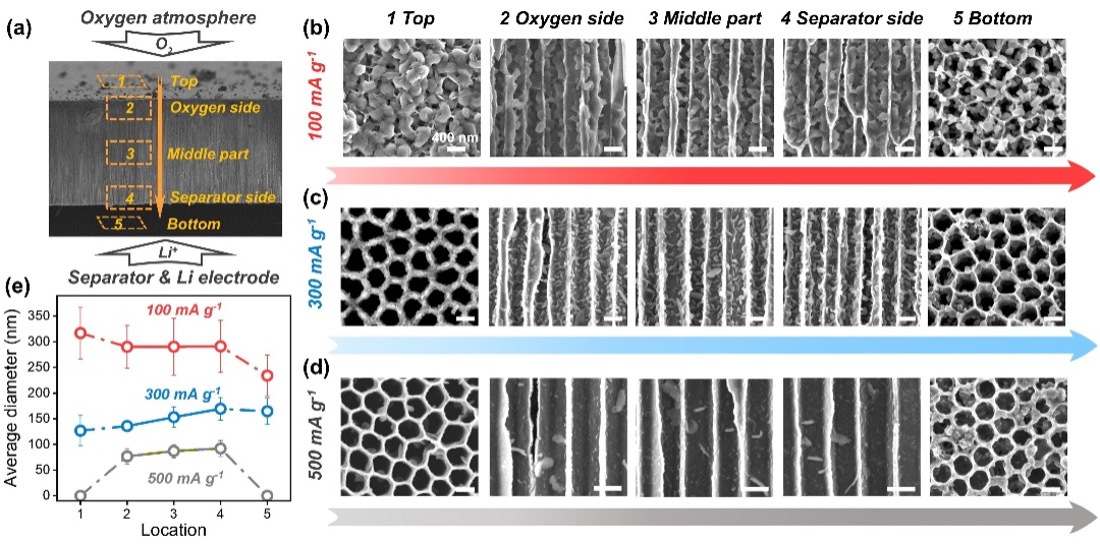
The distributions and sizes of lithium peroxide on the end surfaces of C-AAO electrodes and inside them. (Image by TAN Peng’s team)
The research team designed a special C-AAO electrode that breaks easily yet preserves its distribution of products, enabling Li2O2 observations throughout the entire electrode. Using electrochemical impedance spectroscopy (EIS), the team determined the contributing factor to sudden voltage drop and death at various current densities. Research findings show that, at small currents, channel diameters restrict the growth of toroidal Li2O2, causing electrode blockage. So the sudden death in voltage is associated with a large charge transfer impedance and concentration polarization caused by electrode blockage. While at high currents, the sudden death is attributed to the less significant charge transfer impedance and concentration polarization from the fast electrochemical reactions.
Additionally, in order to find the mechanism of such reactions, the research team carried out detailed analysis on the growth model of Li2O2 on the end surfaces and the interior of C-AAO electrodes. Li2O2 on the end surfaces is found in three toroidal model. The most common one grows “hugging” the wall, forming an incomplete ring. The rest either grows laterally on the surface, or in the form of nuclei, forming on other Li2O2 surfaces. As current density amplifies, toroidal Li2O2 inside the electrode is likely to be covered by its flocculated counterparts, indicating that Li2O2 is produced along the surfaces of the electrode, rather than from disproportionation inside channels. The team proposed a new growth route for toroidal Li2O2, in which Li2O2 formed at the Li2O2/electrode interface during early growth is related to the surface route, followed by lithium peroxide (LiO2) in solution disproportionating around Li2O2 particles, covering the surface route and forming an incomplete ring.
This research provided answers to long-standing questions regarding the mechanism of Li-O2 batteries, as well as insights into further electrodes design.
(Written by SONG Xizhe, edited by ZHANG Yihang, USTC News Center)