Calcium-sensing receptors (CaSRs), widely distributed in tissues and organs such as parathyroid glands, intestines, bones and kidneys, senses the concentration of calcium ions in the blood and maintains the calcium balance in the human body. CaSR is so essential for maintaining blood calcium stability that its abnormal function will lead to various diseases.
CaSR has already been approved as a positive-altering regulator, however, due to the lack of the G protein-coupled complex structure, the complete activation and modulation mechanism of CaSR are still unclear.
A research team led by Prof. TIAN Changlin from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) resolved the structure of the high-resolution three-dimensional complex between CaSRs and the downstream signaling protein Gq for the first time, and revealed the molecular mechanism of the asymmetric activation of the CaSR protein by agonists, positive aliasing modulators, and other molecules.
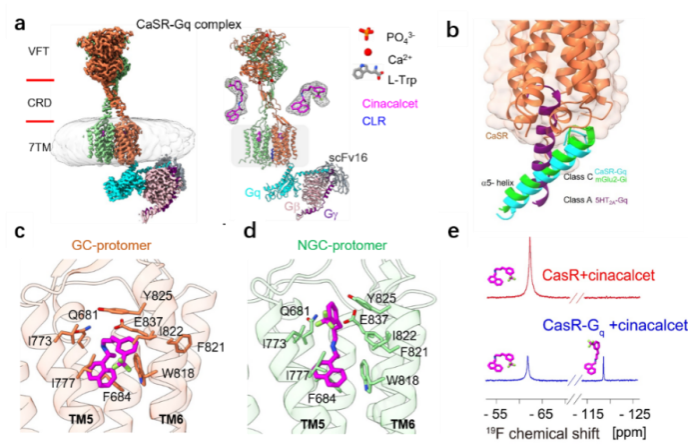
Patterns of asymmetric binding of CaSR to the downstream signaling protein Gq in the presence of agonists and allosteric modulators (Image by USTC)
Their results were published online in Cell Research on Nov. 2.
Researchers obtained the high-resolution cryo-electron microscopy structure of CaSR-Gq complex, which revealed the asymmetric activation mechanism of CaSR binding to Gq and initiating the downstream signaling under the activated state by cell signaling and nuclear magnetic resonance experiments.
Researchers revealed two features of the CaSR-Gq complex structure, the first 3D structure of a C-family G protein-coupled receptor (GPCR) bound to Gq. Firstly, different from the Gq protein binding mode of A- or B-family GPCRs, the α5 helix of the Gq protein was not inserted deep into the transmembrane helices on the intracellular side of the CaSR but rather bound to the receptor cytosolic side in a very shallow binding pocket.
Secondly, the difference in the binding pattern of CaSR-Gq and mGlu2-Gi, GABAB-Gi complexes reflected the specific binding interface between Gq and the receptor.
Researchers revealed for the first time the diverse binding modes of cinacalcet, a positive allosteric modular drug molecule, in the receptor signaling complex. Cinacalcet bound to the extracellular side binding pockets of the two transmembrane structural domains of the dimeric CaSR receptor in two different conformations: extended and bent, respectively. Among them, only the intracellular part of the transmembrane domain of bent-cinacalcet was able to couple to the downstream signaling protein Gq.
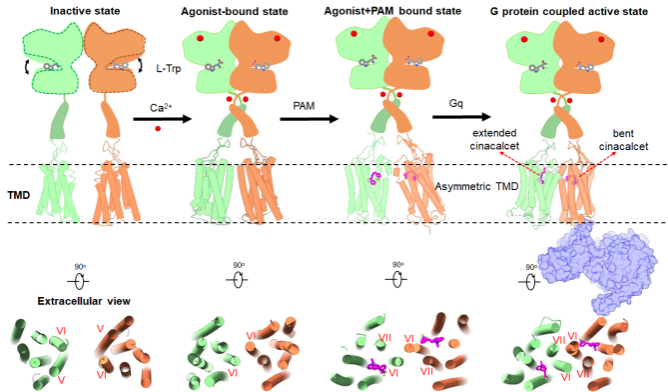
Model of asymmetric activation mechanism of CaSR (Image by USTC)
The researchers have proposed a complete asymmetric activation mechanism of CaSR, which will improve the understanding of the activation mechanism of the C-family GPCRs, and at the same time provide an important theoretical basis for the design of allosteric modulator drugs.
paper link: https://www.nature.com/articles/s41422-023-00892-2
(Written by HUANG Rui, Edited by HUANG Rui, USTC News Center)