A research group led by Professor GAO Minrui from the University of Science and Technology of China (USTC) reports that nickel-tungsten (Ni-W) ratio in Ni-W alloys governed the performance of hydrogen oxidation reaction (HOR). Besides, researchers tuned the unpaired electrons in Ni to adjust the potential of zerocharge (PZC) and hydroxyl adsorption (OHad) capacity of the alloy in alkaline HODs. The results have been published in Angewandte Chemie International Edition.
Hydrogen-oxygen fuel cells are promising candidates in powering electronic vehicles due to their advantages like zero carbon emissions. Also, anion exchange membrane fuel cells excel others in allowing the use of non-platinum metal catalysts. However, in alkaline electrolytes, the kinetics of the anode HOR are reduced by about 100 times compared to those in acidic media. Currently, the activity and stability of Ni-based HOR catalysts still need further improvement.
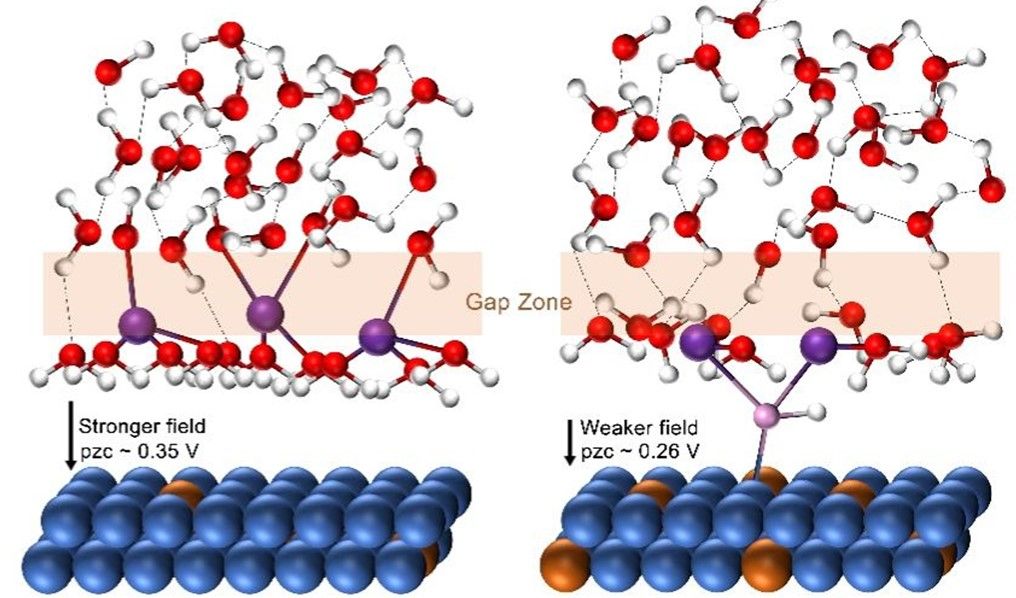
The mechanism of HOR catalyzed by Ni-W alloy. (Image by GUO et al.)
Researchers prepared a Ni-W hydroxide precursor through a microwave thermal strategy, and then prepared a Ni-W alloy by annealing. The HOR activity of Ni-W alloys with different stoichiometric ratios was in the order of Ni19W < Ni9W < Ni3W < Ni4W < Ni17W3, among which the performance of Ni17W3 was the best, superior to commercial platinum carbon catalysts.
Researchers investigated the interfacial water structure by in situ Raman spectroscopy. The results showed that Ni17W3 had the highest proportion of "gap-H2O" molecules, indicating its hydrogen bond network had the best connectivity.
To further verify the role of Ni-W ratio in electronic structure modulation, researchers employed the electron paramagnetic resonance and temperature-dependent magnetization measurements. It was determined that the average number of unpaired Ni 3d electrons in Ni17W3 was 3.7, which was higher than other Ni-W alloys.
The increase in unpaired electrons changed the three-dimensional orbital electron configuration of Ni. In addition, it also reduced the surface work function and PZC and increased the adsorption energy of OH.
Researchers managed to make the PZC and surface electronic states favorable for OHad by alloying Ni with the electronegative W to fine-tune the unpaired electrons of Ni.
The new mechanism revealed in this work has significant implications for breaking through the performance limits of current non-precious metal alkaline HOR catalysts and creating a new generation of non-platinum catalysts.
Paper link: https://doi.org/10.1002/anie.202407613
(Written and edited by HUANG Rui, USTC News Center)