A research team led by Prof. ZHOU Rongbin from the University of Science and Technology of China (USTC) discovered the mechanism by which lysophosphatidylserine (LysoPS) inhibits the anti-tumor activity of natural killer (NK) cells through its receptor. They also proposed a new immunotherapy strategy based on receptor antagonism. Their study was published in Nature Immunology on October 2th.
While immunotherapy has transformed cancer treatment paradigms in recent years, its efficacy remains limited, with response rates below 30%, especially for liver and colorectal cancers. This underscores the pressing need for novel immunotherapy strategies and targets. Conventional natural killer (cNK) cells, known for their crucial role in tumor immunity, face hurdles in penetrating tumor tissues and maintaining their function within the tumor microenvironment. Intriguingly, type 1 innate lymphoid cells (ILC1s), which are abundant in liver and intestine issues, have been found to exhibit both pro-tumor and anti-tumor activities. However, their phenotypic resemblance to cNK cells has hindered the development of targeted interventions, leaving their precise function in tumor immunity unclear.
To uncover this mystery, the team generated tdTomato-GPR34 reporter mice and discovered that GPR34 is highly expressed on ILC1s but not on cNK cells, suggesting that GPR34 could serve as a novel marker to distinguish ILC1s from cNK cells. Through utilizing various subcutaneous tumor models and colorectal cancer (CRC) liver metastasis models, they found that both systemic GPR34 gene deficiency and ILC1-specific deficiency increase the proportion, number, and anti-tumor activity of ILC1s in tumors, subsequently inhibiting tumor growth. This indicates that GPR34 promotes tumor growth by suppressing the anti-tumor activity of ILC1s.
Next, the researchers explored the mechanism by which GPR34 regulates ILC1-mediated anti-tumor immunity. Mass spectrometry revealed that the GPR34 ligand LysoPS accumulates in tumor interstitial fluid. In vitro experiments showed that LysoPS inhibits the ILC1 activation in a GPR34-dependent manner via the cAMP-PKA-CREB pathway. Expression knockout of the LysoPS synthase ABHD16A in tumor cells reduced the levels of LysoPS in the tumor interstitial fluid and inhibited tumor growth. Furthermore, inhibiting the production of LysoPS increased the number, proportion, and anti-tumor activity of ILC1s within the tumors.
Further research revealed that blocking GPR34 with inhibitors could inhibit tumor growth in both subcutaneous tumor models and CRC liver metastasis models. Moreover, the combination of GPR34 inhibitors with anti-TIGIT antibodies could enhance the efficiency of tumor treatment.
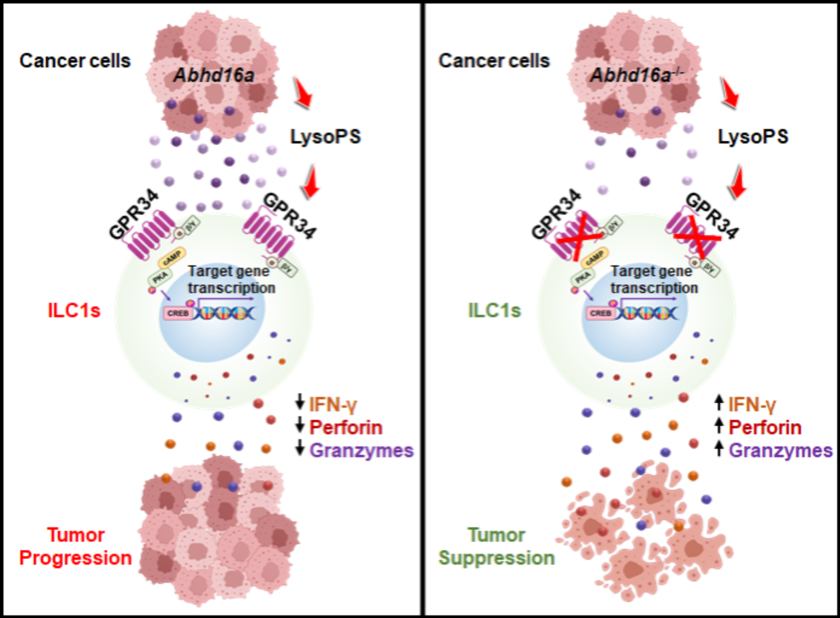
Schematics of GPR34 regulating ILC1 function during tumor progression and suppression. (Image by Prof. ZHOU’s team)
This study identifies GPR34 as a new metabolic immune checkpoint for ILC1-mediated anti-tumor immunity. It provides a novel strategy for immunotherapy by targeting ILC1s, demonstrating potentials for liver and colorectal cancers that respond poorly to current immunotherapies.
Paper link: https://doi.org/10.1038/s41590-024-01973-z
(Written by SHEN Xinyi, edited by ZHANG Yihang USTC News Center)