Research teams led by Prof. SUN Linfeng, Prof. LIU Xin, and Prof. TAN Shutang from the University of Science and Technology of China (USTC) of Chinese Academy of Sciences (CAS), have unveiled the structures of Arabidopsis AUXIN RESISTANT 1 protein (AUX1), and the molecular mechanism by which the protein transports auxin into the cell depending on the proton concentration gradient. The study has been published in Cell on May 15.
Auxin, the first phytohormone discovered in plants, moves directionally within plant tissues to establish concentration gradients, thereby regulating key developmental processes. This directional transport is orchestrated by three protein families: the PIN families, the ABCB families, and the AUX1/LAX families.
Researchers have elucidated the molecular mechanism of Arabidopsis PIN1, revealing how it recognized and exported auxin and how its function was competitively inhibited by NPA. They have also uncovered ABCB19 and ABCB1 were capable of exporting brassinosteroids—and systematically characterized the underlying mechanism. While from the molecule level, auxin influx mediated by the AUX1/LAX transporters still remains uncharacterized.
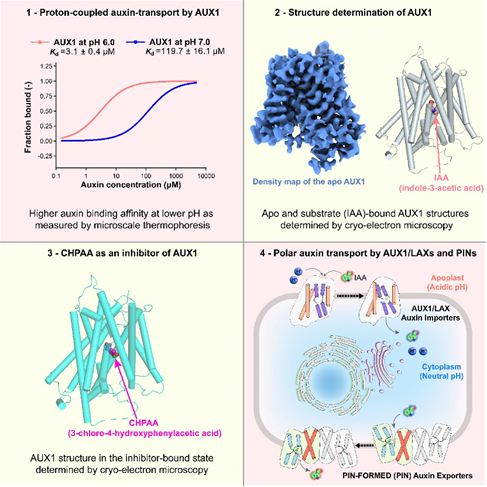
To elucidate the mechanism of Arabidopsis AUX1, the researchers conducted a comprehensive study integrating biochemical assays, structural biology, and computational simulations. They first employed microscale thermophoresis (MST) and isothermal titration calorimetry (ITC) to demonstrate that AUX1-mediated auxin binding and transport are proton-dependent and can be inhibited by the known small molecules 1-naphthoxyacetic acid (1-NOA) and 3-chloro-4-hydroxyphenylacetic acid (CHPAA).
Furthermore, using cryo-electron microscopy, the teams determined high-resolution structures of AUX1 in three distinct states: apo (substrate-free), auxin-bound (IAA), and inhibitor-bound (CHPAA).
These structures revealed, for the first time, the overall architecture of AUX1/LAX family proteins, featuring an inward-facing conformation with 11 transmembrane helices adopting a classical LeuT fold.
In the auxin-bound structure, several key residues involved in IAA recognition were identified—most notably His249 (H249), which underwent a marked side-chain reorientation upon substrate binding.
The functional relevance of these residues was confirmed through site-directed mutagenesis, biochemical assays, and plant physiological experiments.
To further investigate the role of H249 in substrate recognition and proton coupling, the teams collaborated with Prof. ZHU Lizhe’s team at the Chinese University of Hong Kong (Shenzhen) to perform molecular dynamics simulations.
Comparative analysis of the IAA- and CHPAA-bound structures showed that, while both ligands occupied a similar binding pocket, they exhibited distinct interaction patterns. These differences provided mechanistic insights into how CHPAA inhibited AUX1-mediated auxin transport.
Mechanism of AUX1-mediated auxin influx. (Image by USTC)
The study has revealed for the first time the molecular basis of auxin import mediated by the AUX1/LAX protein family in plants, which are essential for understanding plant growth and development, and future applications in agriculture.
Paper link: https://doi.org/10.1016/j.cell.2025.04.028
(Written by SHEN Xinyi, edited by HUANG Rui, USTC News Center)