Researchers revealed new mechanism for ion selection of NaK ion channel by solid state NMR (Nuclear Magnetic Resonance), single-channel electrophysiological and molecular dynamics simulation. The work was published on Nature communications on February 19, entitled “A single NaK channel conformation is not enough for non-selective ion conduction”. The finding is a combined effort by Prof.TIAN Changlin from University of Science and Technology of China and Adam Lange and Sun Han from the Leibniz Institute of Molecular Medicine, Germany.Ion channels is a kind of special hydrophilic microporous channel on the cell membrane, it determines the excitability and conductivity of cells by forming action potential and gradient potential on the cell membrane. Most ion channels selectively permeate different ions, but some ion channels still can pass several ions non-selectively. Based on the KcsA potassium ion channel structure, researchers proposed a static mechanism model for "ion selectivity of the K+ channel through hydration ion coordination of the backbone C = O in the selective filter (Selective filter)", which is now widely agreed. However, in recent years, the high-resolution X-ray crystal structure shows that the static channel structure of the NaK ion channel when bound to different ions (such as Na+, K+, Rb+) is exactly the same, and this fact can’t explain how it recognizes and permeates these ions.
Prof. TIAN Changlin’s group reassembled non-selective channel NaK into the phospholipid bilayer membrane. Together with Adam Lange’s group, they obtained the peaks assigned to different metal cations high resolution Solid-state NMR spectra through the magic angle spin solid NMR method. NMR spectroscopy data showed two conformations of NaK channel in physiological environment, potassium ion is selected to combine with one of them, and sodium ion, the other. The two parties further delineated the structural differences between the two conformations by measuring the distance between the solid-state nuclear magnetic resonance and the molecular dynamics simulation, and confirmed that the two conformations have high selectivity to K+ and Na+ respectively. The results show the importance of protein conformational diversities and conformational shifts in protein function, suggesting a new mechanism of ion channel selectivity.
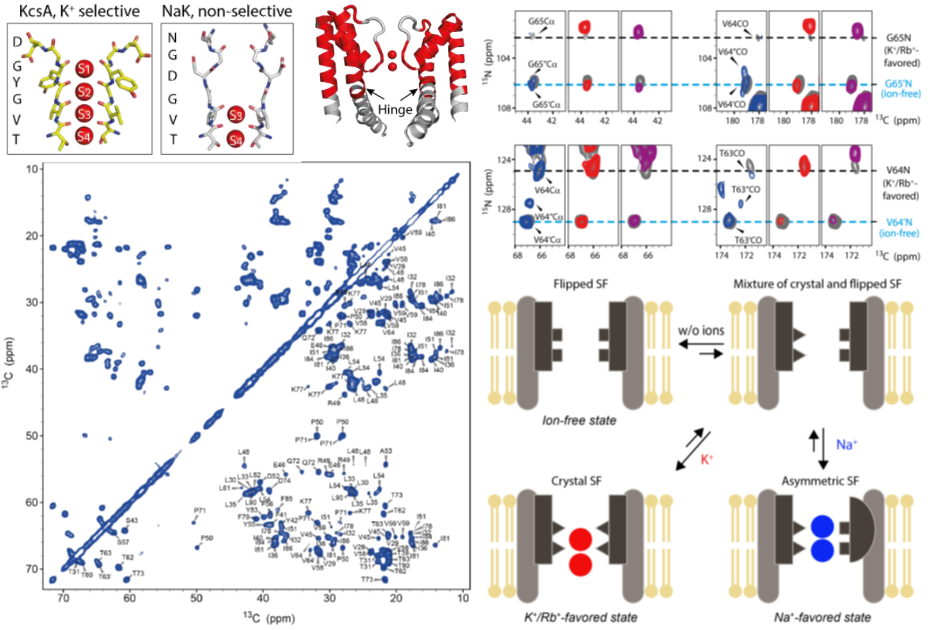
New mechanism for NaK ion channel selectivity, Image by Tian Changlin
The research was funded by the Fund Commission, Ministry of Science and Technology, Chinese Academy of Sciences and Hefei University Science Center.